The availability of rhizosphere nutrients affects the role of saprophytic bacteria and mycorrhiza in litter decomposition and enzyme activity.
Compilation: Wei Ke Meng Suoya, Editor: Wei Ke Meng Suoya, Jiang Shunyao.
Wei Kemeng’s original micro-essay, welcome to forward and reprint, and the source of the reprint must be indicated in WeChat official account.
guide reading
Professor Tian Xingjun from School of Life Sciences, Nanjing University and others published an article entitled "Synergy of saporphs with mycorhiza for little decomposition and hotspot formation dependencies on nutrient availability in the sphere" in Geoderma on December 27th, 2021. Plants can obtain and recycle nutrients from soil organic matter, and these dynamics depend on highly diverse rhizosphere microorganisms. Although the functions of many microbial groups have been determined, how different groups interact to influence biogeochemical processes is still poorly understood. In this study, the effects of the interaction between saprophytic bacteria and arbuscular mycorrhizal fungi (AMF) on the decomposition of alfalfa litter and the spatial distribution of enzyme activities in poor soil and nutrient-rich soil were studied by zymogram analysis.In poor soil, compared with single inoculation, co-inoculation of humic bacteria (Alcaligenes faecalis) or saprophytic fungi (Phanerochaete chrysosporium) with AMF(Rhizophagusi rregularis) can decompose litter faster and make the hot spot area of rhizosphere enzyme larger..The interaction between AMF and saprophytic bacteria is stronger than that with saprophytic fungi. The synergistic effect of saprophytic bacteria and AMF on litter decomposition and enzyme hot spot formation is weaker in eutrophic soil than in barren soil. On the contrary, in eutrophic soil, the co-inoculation of saprophytic fungi and AMF reduced litter decomposition.. PLS-PM analysis shows that,Faster litter decomposition and larger enzyme hot spots can promote plant growth.. Our results show that,There is an important synergistic relationship between these two microorganisms, which plays an important role in promoting plant growth in poor soil.. Among them,AMF can promote litter mineralization by increasing the biomass and enzyme activities of saprophytic bacteria (nitrate reductase, urease and cellulase).. At the same time, saprophytic bacteria increased the colonization rate of AMF and the hot spot of enzyme activity. These hot spots in turn expand the nutrient activation area around the roots-rhizosphere. In a word, the synergistic effect between saprophytic bacteria and AMF on litter decomposition and the formation of enzyme hot spots may be an important mechanism to promote plant adaptability under the condition of low soil nutrient availability.
sb/sth attracting attention 1. Arbuscular mycorrhizal fungi (AMF) can increase litter decomposition by stimulating saprophytic bacteria in malnourished soil. 2. inoculation with AMF can enlarge the hot spot area of rhizosphere enzyme activity by 13% ~ 400%. 3. In the soil with poor nutrients, AMF is more important for plant growth than in the soil with sufficient nutrients.
Key words:Litter decomposition, rhizosphere process, nutrient cycle, plant-soil-microorganism interaction, soil zymogram
Paper ID
originName:Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere
translatename:The synergistic effect of saprophytic bacteria and mycorrhiza in litter decomposition and enzyme hot spot formation depends on the availability of rhizosphere nutrients.
journal:Geoderma
IF:6.114
Publication time:2021.12.27
Communication workBy:Professor Tian Xingjun
Communication author unit:Nanjing University School of Life Sciences
DOI number:10.1016/j.geoderma.2021.115662
Experimental design
Medicago sativa L., a common host plant of AMF, was selected as the experimental plant and planted on two kinds of soil (nutrient-rich soil and barren soil). A total of 6 inoculation treatments (Control, not inoculated; Sapr-Bact, inoculated with saprophytic bacteria A. faecalis; alone; Sapr-Fungi, inoculated with saprophytic fungus p.chrysosporium alone; AMF, inoculated with R. irregularis; alone; Sapr-Bact/AMF, A. faecalis and R. irregularis were co-inoculated; Sapr-Fungi/AMF, co-inoculation of P. chrysosporium and R. irregularis). A total of 108 samples (6 inoculums × 2 nutritional levels × 3 sampling times × 3 replicates). After planting for 3 months, samples of plants, litter and soil were taken every month to determine the nutrient content. Microbial biomass, AME colonization, soil zymogram analysis and litter decomposition were determined.

result
Table 1. Chemical properties of litter and soil
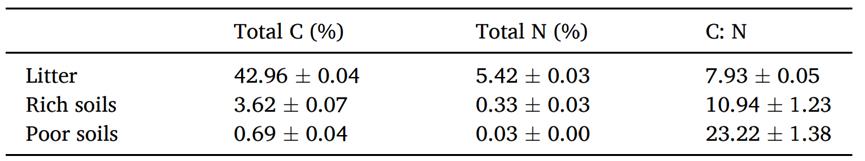
Mean and standard deviation (n = 3)
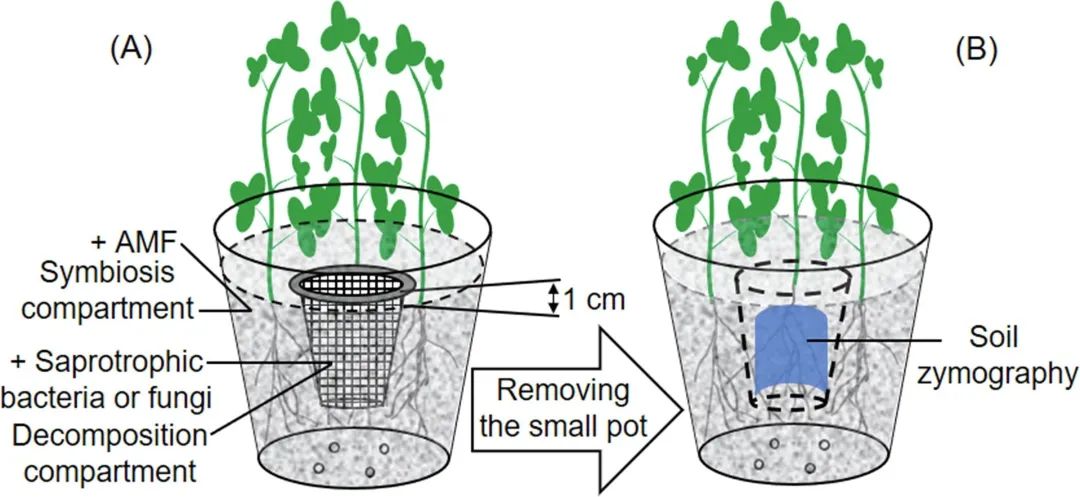
Figure 1. Design of experimental miniature basin. Nylon mesh (aperture 30 μm) separates the big basin from the small basin. AMF is added to the big pot, and saprophytic bacteria or fungi are added to the small pot. The top edge of the small pot is 10mm above the soil surface, which makes the pot easy to take out. The spatial distribution of enzyme activity on the surface of the inner hole of the big basin after removing the small basin was determined by zymogram.
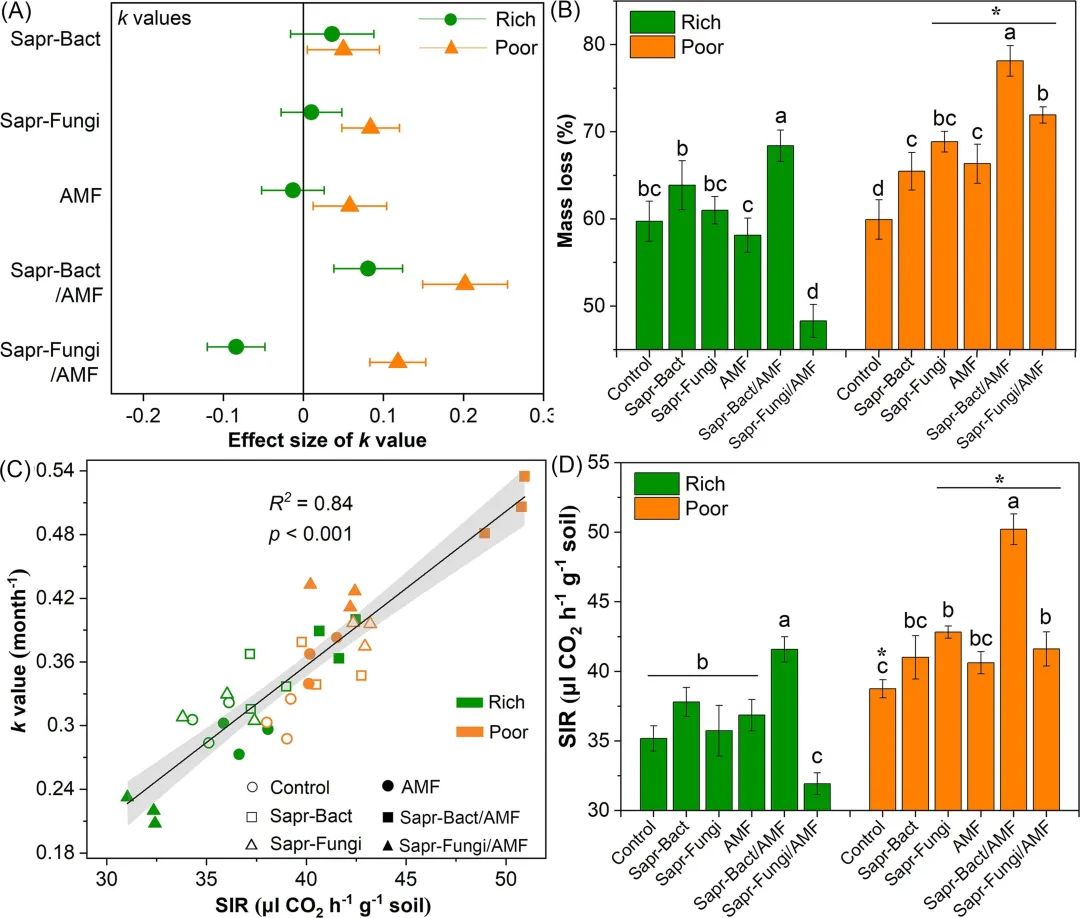
Fig. 2. The influence of litter decomposition rate (K value) (a), the mass loss of alfalfa litter (b), the correlation between substrate-induced respiration (SIR) and litter decomposition rate (c), and the SIR value (d) of inoculation amount at two soil nutrient levels after decomposition for 3 months. The value in (a) is the average of the influence of inoculation on the K value (n = 3) and the 95% confidence interval. Not exceeding the zero line indicates that the impact is significant. The values (b and d) are mean and standard deviation (n = 3 and 9). The letter indicates that there is a significant difference between the two kinds of soils under the same soil nutrient level, and the asterisk indicates that there is a significant difference between the two kinds of soils under the same microbial inoculation (p <0.05). (Control, not vaccinated; Sapr-Bact, inoculated with saprophytic bacteria A. faecalis; alone; Sapr-Fungi, inoculated with saprophytic fungus p.chrysosporium alone; AMF, inoculated with R. irregularis; alone; Sapr-Bact/AMF, A. faecalis and R. irregularis were co-inoculated; Sapr-Fungi/AMF, co-inoculation of P. chrysosporium and R. irregularis).
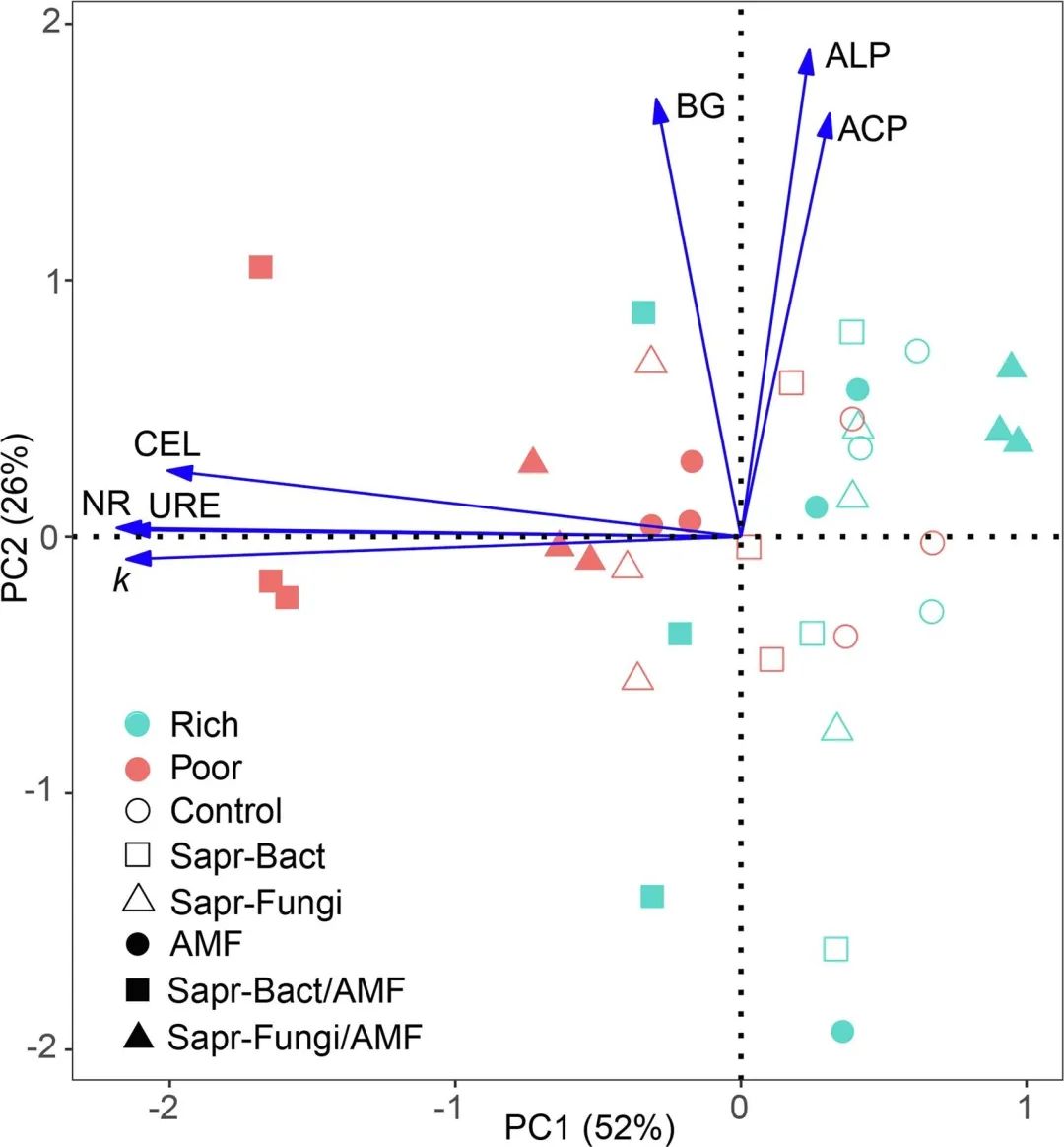
Fig. 3. Principal component analysis (PCA) of enzyme activity (arrow) and decomposition rate (k) between different treatments. Characters are centered and standardized before sorting. (NR, nitrate reductase; URE, urease; ACP, acid phosphatase; ALP, alkaline phosphatase; BG, β -glucosidase; CEL, cellulase).
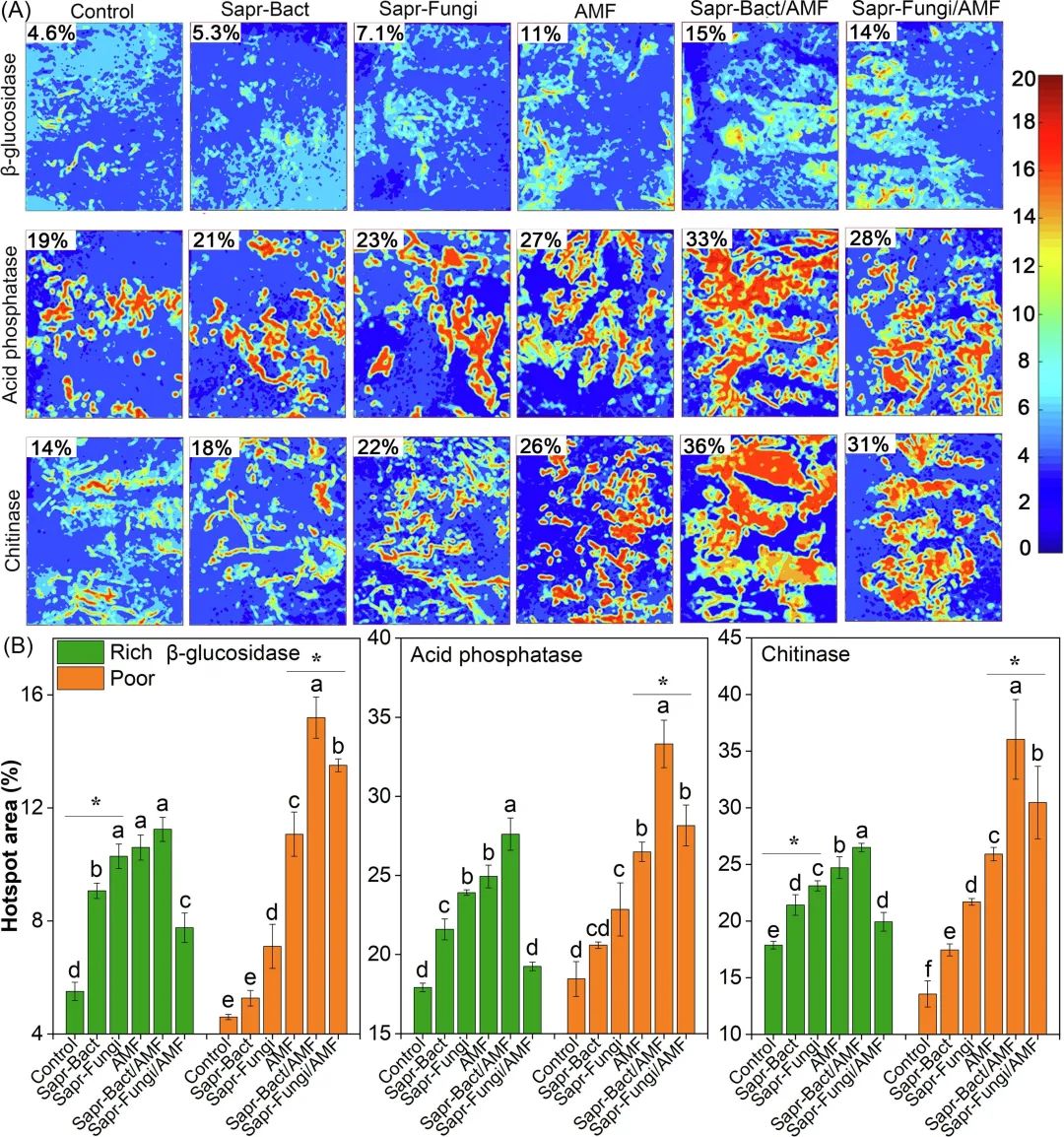
Fig. 4. The zymogram of β -glucosidase activity, acid phosphatase activity and chitinase activity in poor soil three months after transplantation. The number in the upper left corner of each zymogram shows the percentage of hot spots (A). Color scale indicates enzyme activity (pmol mm-2h-1). Hot spot area percentage (b) of each inoculation point of the two soils after decomposition for 3 months. The values are mean and standard deviation (n = 3). The letter indicates that there is a significant difference between the two kinds of soils under the same soil nutrient level, and the asterisk indicates that there is a significant difference between the two kinds of soils under the same microbial inoculation (p <0.05).
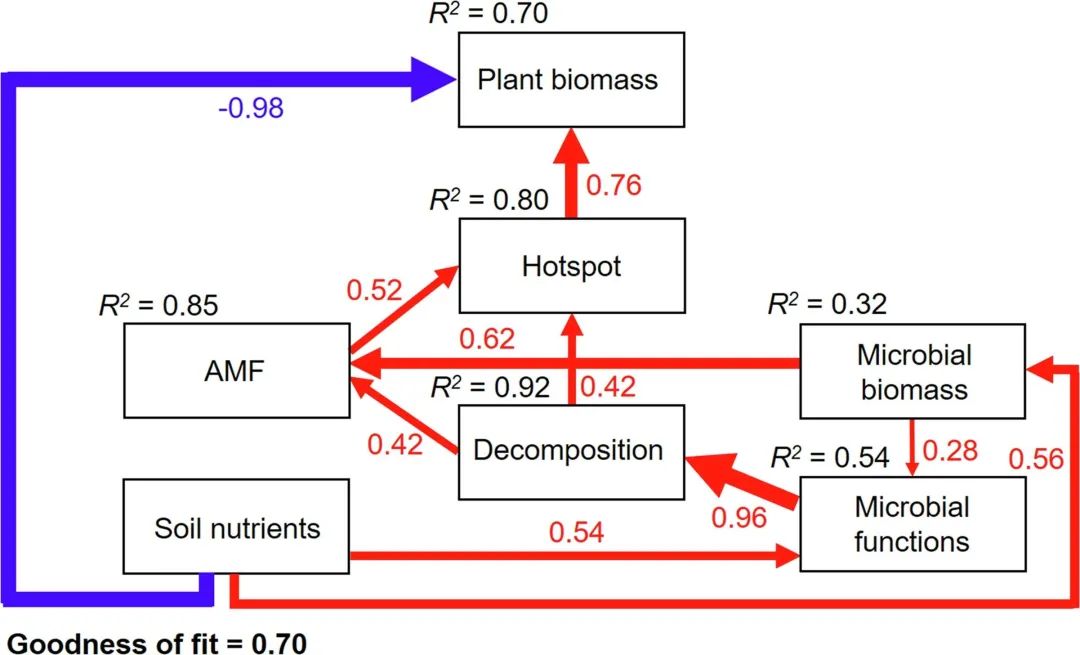
Fig. 5.PLS-PM shows the direct or indirect influence between soil nutrient level, AMF colonization rate, microbial biomass and microbial decomposition of litter, plant biomass and hot spots. The width of the arrow is proportional to the strength of the path coefficient. Blue and red arrows indicate negative and positive effects, respectively. The arrow indicates the significant effect (p <0.05). The numbers on the arrows represent significant standardized path coefficients. The R2 value represents the explained variance. Microbial functions include: nitrate reductase and urease activity; Plant biomass includes: aboveground biomass and root biomass; Enzyme activity hotspots include β -glucosidase activity, acid phosphatase activity and chitinase hotspot.